Register now for our 2-day courses in 2026
27 - 28 May | Early-Bird fee: €2495 + VAT* | Course fee: €2695 + VAT
The upcoming course will take place in the Netherlands in May 2026. The maximum number of attendees is 20, so make sure to register in time. Use the Register Now button to download the registration form. For more information, contact Kantisto.
Kantisto also offers on-site consultancy, implementation support, and courses with content tailored to your needs.
* Contact us now to take advantage of the early bird discount and secure a reduced fee! Early-Bird offer applies up to 3 months prior to the course start date. 
Enhancing Analytical Method Development and Knowledge Sharing
Do you want to break down the guidelines (ICHQ14, ICHQ2(R2), and USP <1220>) into a practical AQbD workflow with tools? Do you want to take the opportunity to capture the scientific knowledge built during analytical method development and better share this knowledge internally or with the regulatory bodies? The AQbD tools will facilitate efficient and risk-based development and validation of analytical methods and for keeping product and process control strategies scientifically sound and fit-for-purpose. This course demystifies and presents AQbD in a digestible way.
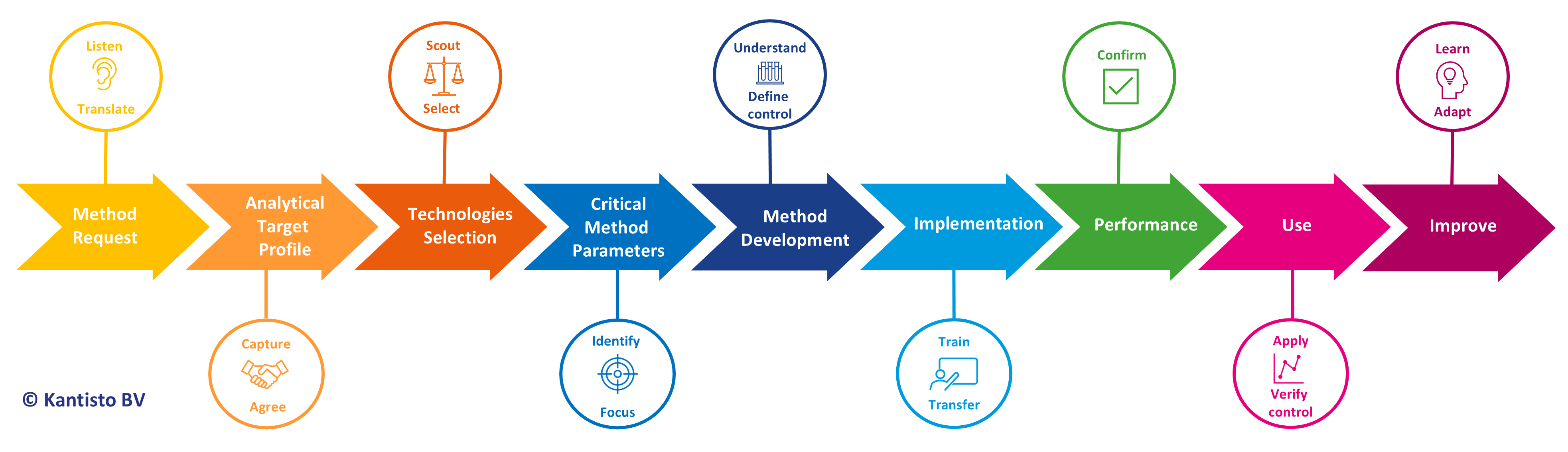
The AQbD process starts with defining objectives and a handshake between project and analytical development. It includes identifying critical method parameters, risk-based decision making, and continuously following and improving the procedure. The resulting method is better understood, more robust, and in control, thus reducing the need for troubleshooting and costly reanalysis. The knowledge and decisions made during development are captured, shareable, and reusable throughout the whole pharmaceutical life cycle.
See here for the 2026 brochure.
For whom
This course aims at managers and scientists who want to apply a scientific AQbD approach in their daily work or gain knowledge on how to start implementing AQbD within their organization.
Course content
- Background of Analytical Quality by Design
- Detailed training on the AQbD flow and the specific modules:
- Analytical Request and Analytical Target Profile (ATP)
- Technologies selection
- Critical Method Parameters (CMP)
- Method Development, including Design of Experiments
- Method Implementation
- Method Verification
- Procedure life cycle management
- Analytical Request and Analytical Target Profile (ATP)
- How to implement AQbD within your organization
- Exercises for each module
- Rapid learning cycles by combining theory and exercises
- Discussions in an open and positive environment
- Sharing examples and experiences from industry
- Templates you can adapt for your own organization
Learning outcome
This 2-day course will provide time to network and discuss with fellow scientists. After this course you have learned how to apply AQbD in your daily work and are motivated to (further) implement this approach within your organization. A training certificate, practical templates and hand-outs will be provided.
The trainers
The training will be provided by Dr. Cari Sänger - van de Griend (Linkedin) and Dr. Ewoud van Tricht (Linkedin). We share years of experience applying and implementing the principles of AQbD within (bio)pharmaceutical companies. By doing so, we improved the coherence between analytical methods and the process and quality profile of the product, strengthening the concept of “right analytics at the right time”. AQbD increased our level of control and understanding of the analytics as well as reduced our development costs and time. More information: About Kantisto.

Feedback from previous courses
- AQbD demystified and presented in a digestible way!
-
This course is an excellent way to learn the AQbD concepts.
- Everyone in our organization should do this course. Many applicable solutions were discussed in a comprehensible way that we can apply immediately.
- The course covers the fundamentals of the AQbD and it gives the courage to try it for yourself.
- My basic knowledge about AQbD was mostly on DoE robustness. I now feel secure that the course helps me to support my group in every AQbD subject.
- The course made me better understand the whole workflow and the relationship between all different steps.
- The rhythm of the training was perfect! Well fragmented, good timings, exercises at the right time.
- The exercises in this course took me over the fear of starting to use AQbD myself.
- Many practical examples that give grip on the topic. The exercises clearly demonstrated and underlined the need for an AQbD mind set.
- I liked the exercises and the playfulness of them and the teachers.
- The teachers had a good balance, they have lots of experience and complement each other.
- It is very inspiring to see the course leaders talk so enthusiastically about their field.
- There was plenty of opportunity to ask questions and the speed of the training was flexible and adapted to our uptake.
- I have really enjoyed this course and look forward to use all that I’ve learned to my method development at work. Thank you very much!
- I’m so exited to go back to the lab to develop a new method with all I learned!
Nine Steps to Master Analytical Quality by Design (AQbD):
- Free download of the PDF
Key words: ICH Q14, MLCM, AQbD training, Analytical procedure development, systematic approach, life cycle management


